GLP-1 medications have quickly moved from niche diabetes treatments to widely prescribed drugs for weight management without racing or physical exercises and for metabolic health. Their rapid adoption has been fueled by social media visibility, telehealth prescribing, and growing consumer demand for medical solutions that promise long-term lifestyle change. But as usage expands, so does scrutiny. A growing wave of GLP-1 drug lawsuits is drawing attention to how health technology systems monitor safety, communicate risk, and protect consumers.
A clinical audit of 37,323 GLP-1 prescription records identified 1,654 prescribing errors, representing an error rate of approximately 4.4%. This finding highlights practical oversight challenges within digital prescribing workflows, particularly as GLP-1 medications scale rapidly across telehealth platforms and decentralized care models.
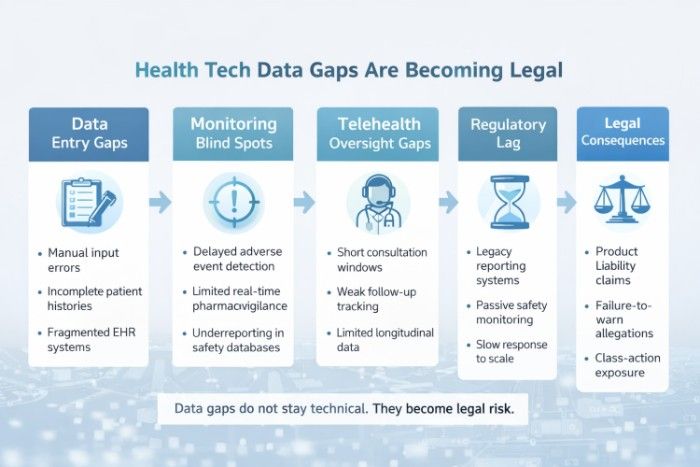
At the center of this debate is oversight not only of pharmaceutical products, but of the digital health infrastructure that tracks outcomes, reports adverse effects, and ensures patients are appropriately informed. These cases are not just about individual injuries. They are revealing systemic gaps in how health data is collected, analyzed, and acted upon across the healthcare ecosystem.
Key Takeaways
- GLP-1 drug lawsuits highlight issues with healthcare monitoring and safety oversight during rapid adoption.
- A clinical audit revealed a 4.4% error rate in GLP-1 prescriptions, underscoring flaws in digital prescribing.
- Lawsuits expose gaps in health tech data management, revealing systemic risks that affect consumer safety.
- Consumers increasingly demand transparency regarding risks, impacting trust and legal accountability for health brands.
- Healthcare regulation must adapt to include oversight of technology, beyond just pharmaceuticals, to ensure consumer protection.
Table of Contents
Rapid Adoption Has Outpaced Monitoring Systems
The pace at which GLP-1 drugs entered the mainstream challenged regulators, providers, and technology platforms. Almost immediately, prescription volumes rose before the possibility of large-scale assessment in long-term real-world studies. Post-market surveillance has failed to keep pace with large-scale and off-label use, as well as with new patient groups, despite clinical trials providing a safety base.
Sarah N. Westcot, Managing Partner at Bursor & Fisher, P.A., points to this disconnect between innovation and oversight:
“Many of these lawsuits stem from a lack of timely warnings and incomplete risk disclosures. When drugs scale faster than the systems designed to monitor them, consumers can be left without the full picture needed to make informed medical decisions.”
Her observation reflects a broader problem: technology is making care easier to access, but has not kept pace with accountability.
Health Tech Data Gaps Are Becoming Legal Fault Lines
Modern healthcare depends heavily on digital systems, electronic health records, adverse event databases, AI-driven analytics, and patient portals. When these systems fail to flag emerging risks or communicate patterns effectively, problems escalate quietly until litigation forces them into the open.
Public databases such as the U.S. Food and Drug Administration’s adverse event reporting system (FAERS) rely on timely, accurate reporting from manufacturers and providers. However, underreporting and delayed analysis can mask trends that would otherwise prompt earlier intervention. As lawsuits emerge, attorneys increasingly examine whether available data could and should have been acted upon sooner.
Dr. Nick Oberheiden, Founder at Oberheiden P.C., emphasizes that regulatory exposure often begins with data oversight failures:
“In federal investigations and large-scale litigation, we consistently see that the issue isn’t a lack of data it’s how that data was monitored, escalated, or ignored. Health tech oversight has become a central compliance issue, not just an operational one.”
Consumer Trust Is Now Tied to Transparency
Beyond regulatory compliance, GLP-1 lawsuits are transforming consumers’ perceptions of health brands’ credibility. Patients today are not only in need of transparency regarding benefits, but also regarding risks, adverse effects, and long-term effects. Where such expectations clash with partial disclosure, reputational losses can go way beyond the courtroom.
These activities are being followed by brands competing in the nearby wellness and health sectors. According to Gerrid Smith, Founder & CEO of Fortress Growth, trust is no longer a separate concept from data responsibility:
“Consumers are more informed and more skeptical than ever. When health outcomes don’t align with expectations, they look for accountability. Companies that prioritize transparency and responsible data practices are better positioned to maintain trust when scrutiny increases.”
This change is significant because consumer perceptions are gaining increasing power in legal proceedings. Not only can clinical evidence support the lawsuit, but patterns of dissatisfaction, confusion, or perceived omissions can also gain a foothold.
Oversight Must Extend Beyond Pharmaceuticals
Among the greatest lessons that emerged from the GLP-1 litigation is that the drug itself can never be the subject of oversight. The role of telehealth platforms, digital marketing platforms, and prescribing algorithms in shaping the patient experience is not overlooked. Liability arises if a failure occurs at any point in the risk communication chain.
The domain of health technology regulation has expanded to encompass consumer protection, data privacy, and advertising standards. Courts and regulators are starting to analyse whether healthcare models that use technology actually provide sufficient protection to users, or whether the speed and scale are compromising due diligence.
The Future of Health Tech Accountability.
The lawsuits on GLP-1 drugs are most likely to foreshadow the future. Overseeing healthcare will increase as it becomes more digitized. The companies are expected not only to invest in innovation but also in systems that can detect early warning signs, communicate the risk, and respond quickly when problems arise.
Enhanced supervision does not imply the lack of speed. It is about coordinating technology, regulation, and ethics to allow innovation to scale responsibly. Health technologies that emphasize data accuracy, monitoring, and transparency will be better positioned to withstand regulatory scrutiny and safeguard the consumers they serve.
Conclusion
The ROAR of GLP-1 lawsuit claims highlights an important fact: healthcare innovation today needs the same regulatory novelty. Criminal cases are revealing weaknesses in monitoring systems, with data disclosure failures leaving end users at a loss. With the demanding standards set by regulators, courts, and the public, health technology oversight will cease to be optional; it will become fundamental.
To those companies in this space, the message is well understood. The future of oversight is in the hands of those who see it not as a limitation but as an essential element of trust, compliance, and long-term success.











